leading the future of nucleic acid manufacturing and analysis

your GMP partner
Nucleic Acid Manufacturing and Analysis Solutions
BioSpring Empowers Your Therapeutic Innovations
Customized Solutions for Nucleic Acid Manufacturing and Analysis - From Research to Commercial Applications
Welcome to BioSpring, your trusted partner in advancing nucleic acid-based therapies. With nearly three decades of experience, we specialize in nucleic acid manufacturing and analytical services that are vital to the success of your drug development programs. From preclinical research to full-scale commercial production, we offer unparalleled quality, flexibility, and regulatory support, ensuring your therapies reach patients faster and with greater efficiency.
For all stages of drug development
Comprehensive Manufacturing Services
At BioSpring, we provide end-to-end manufacturing solutions for nucleic acids, covering everything from small-scale research production to large-scale commercial batches. Our FDA-inspected, GMP-compliant facilities guarantee the highest quality, while our innovative technologies ensure your programs are prepared for every phase of clinical development. Whether you're working with siRNAs, ASOs, guide RNAs, or other nucleic acids, our tailored processes are designed to meet your exact needs.
Covering the entire drug development process.

Research & Discovery
- Manufacturing for Research & Development, lead screening, and lead optimization
- Custom manufacturing solutions and chemistries
- High-throughput manufacturing

Preclinical
- Early preclinical, mid-scale batch manufacturing
- Regulatory tox batch manufacturing
- Process development & scale-up

Clinical & Commercial
- Commercial manufacturing and process validation
- cGMP manufacturing for clinical phase I-III (ICH Q7)
- Process transfer and optimization
Ensuring precision and compliance
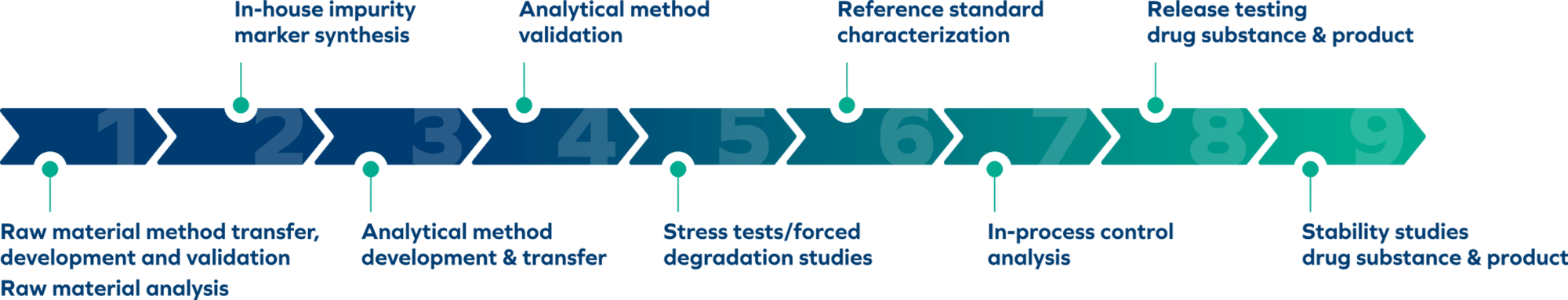
Cutting-Edge Analytical Services
For Drug Substance & Drug Product
- Release testing
- Stability studies (ICH-compliant)
- Raw material method transfer, development & validation
- Raw material analysis
- In-house impurity marker synthesis
- Analytical method transfer, development & validation
- Stress tests/forced degradation studies
- Reference standard characterization
- In-process control analysis
The Nucleic Acid Company
About BioSpring
BioSpring is a leading industry expert in nucleic acid science and technology, specializing in high-quality cGMP manufacturing and analytics for commercial programs and clinical phases I - III, in addition to preclinical material supply and diagnostic manufacturing. We are a privately owned company of over 650 employees committed to providing expert support through all phases and regulatory filings, including highly flexible and customizable solutions to accelerate therapeutic programs through the entire drug development lifecycle.
Headquartered in the center of Europe, in Frankfurt, Germany, we are an international company with a subsidiary in the United States and a local presence in Japan. Our global clients can rely on our quality and expertise, guided by our passion for innovation and three decades of experience. Our aim is a highly collaborative partnership to unleash the full potential of our clients’ technology, making great strides and breakthroughs possible.
650
Employees
40
Nations
∞
Ideas

