High-quality solutions for your therapeutic genome editing needs

A new era of medicine
mRNA Manufacturing
cGMP IVT mRNA Manufacturing
What is mRNA?
Messenger RNA (mRNA) is a carrier molecule that moves information encoded for a protein from a chromosome within a nucleus to the translation site in the cytoplasm. mRNA’s capacity to employ a cell’s translational machinery positions it as a universal blueprint for protein production, dictating the production of proteins that are essential for various biological functions.
Since the mRNA vaccine race to market with the advent of COVID-19, a new generation of mRNA therapeutics has sparked interest in the biotechnology world. Its rapid development and adaptability make mRNA a powerful tool in addressing a wide range of health conditions.
How is mRNA used?
mRNA can be synthesized to instruct cells to produce specific proteins, which can be used to treat or prevent diseases. This approach offers a flexible platform for addressing a wide range of medical conditions and for the development of novel therapies. mRNA technology allows for the development of targeted treatments for genetic disorders, the creation of cancer vaccines, and the enhancement of the body's immune response to infections. In gene editing, mRNA can be engineered to encode specific proteins, such as Cas9, which, when used alongside guide RNA, facilitates precise and targeted modifications to the genome.
mRNA Services Overview
BioSpring offers end-to-end services for custom in vitro transcription (IVT) mRNA production, offering comprehensive and integrated services for mRNA manufacturing, upscaling, and analytics. Our mRNA manufacturing solutions are designed to meet the highest quality standards to support early discovery and preclinical programs through clinical and commercial programs.
- Your partner for all stages: Research-grade through cGMP manufacturing (ICH Q7)
- Adapting to your needs: Process development, scale-up, and tech transfer
- >18 years of experience in clinical and commercial manufacturing of RNA
- Highly experienced in large-scale downstream processing for cGMP programs involving HPLC purification
Preclinical mRNA Manufacturing
- Synthesis and purification of research- and preclinical-grade mRNA, starting at µg batch sizes
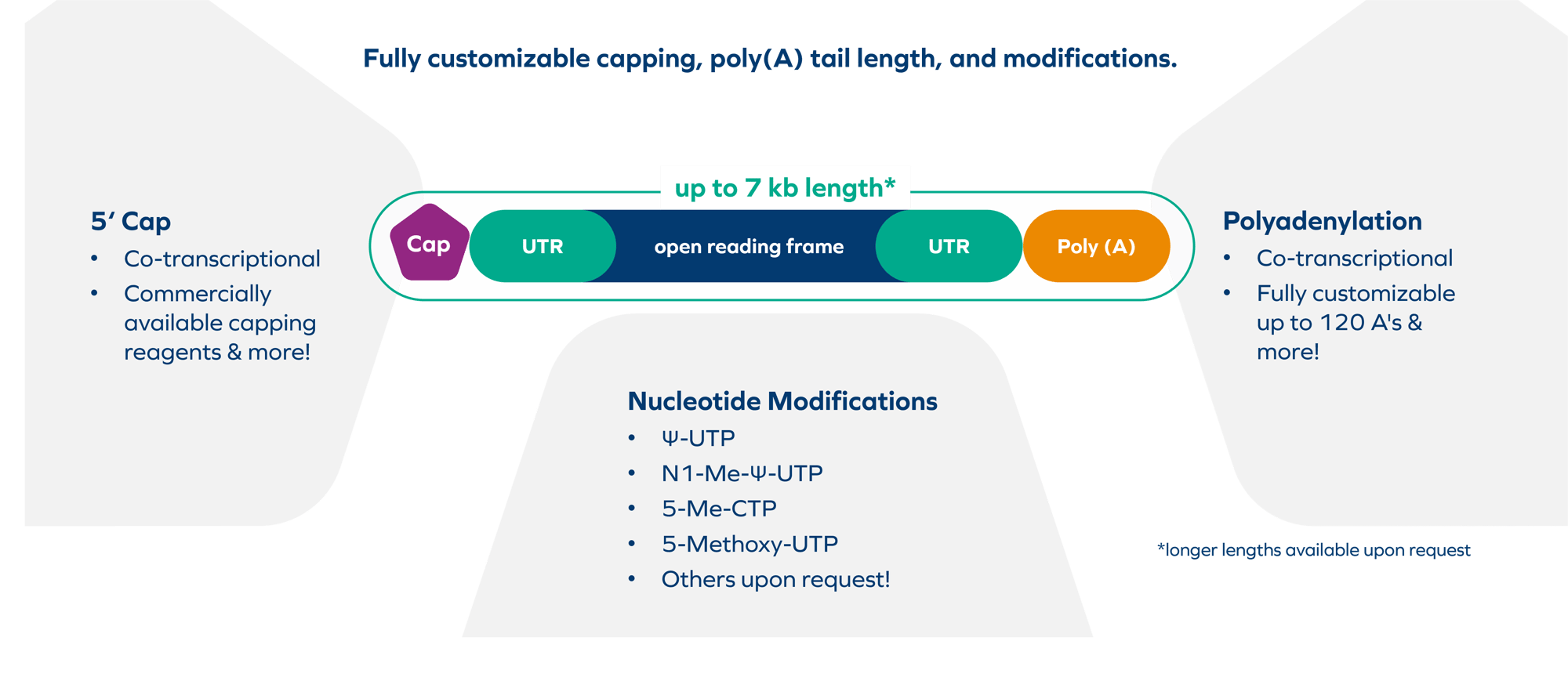
- Fully customizable options for capping, poly(A) tail length, and modifications
- Highly pure mRNA through cutting-edge HPLC technology
- Manufacturing timelines of approximately 14 days (from receipt of DNA template)
cGMP-Grade mRNA Manufacturing
- State-of-the-art facilities with ISO-classified cleanrooms
- Flexible batch sizes up to 250 g and fully customizable options for capping, poly(A) tail length, and modifications
- Powerful platform for flexible process development
- Highly pure mRNA through cutting-edge HPLC technology
- Regulatory support
Our Capabilities
Ensuring accuracy and integrity
Analytical Services for mRNA
Your program benefits of our customizable analytical solutions ensuring the highest quality and stability of your mRNA products. We offer an extensive suite of services, including:
- High-resolution analysis: Single analysis, high-resolution mass spectrometry (MS) for capping and poly(A) tail integrity, and ELISA for dsRNA detection
- Single assay for capping efficiency and poly(A) tail length by HRMS
- High-fidelity in-house sequencing using NGS
- dsRNA measurements with superior sensitivity
- Various ribonucleic acid quantification methods available
- Method validation, characterization, release testing, and stability studies
Excerpt of R&D-grade release testing scope:
- Integrity by CE
- Purity
- Impurities
- UV absorbance
- mRNA concentration
- Endotoxin (optional)

Why Choose BioSpring?
Comprehensive Services
At Every Stage
At BioSpring, we provide the full suite of services to support your project from start to finish. Whether you’re in the early phases of your therapeutic nucleic acid program or looking to scale up production, we’ve got you covered. Our expertise ensures seamless transitions through each stage, so your project progresses smoothly, from initial concept to the commercial market.
Why Choose BioSpring?
Flexible Timelines
Ready When You Are
We understand that timing is everything in drug development, that’s why our process is designed with flexibility in mind. We work closely with our clients to meet their goals efficiently and on schedule. No matter where our clients are in their development cycle, we ensure that our services align with their unique timeline.
Why Choose BioSpring?
Client-Centric Services
Project Management and Regulatory Support
With your success as our priority, our dedicated project managers and subject matter experts provide strategic and technical support from your initial inquiry to product delivery and beyond. Our regulatory team offers comprehensive support, handling everything from technical writing and dossier maintenance to thorough reviews of submissions like IND, NDA, BLA, and DMF filings, ensuring all documentation meets regulatory standards. Count on us to be by your side throughout the entire project lifecycle, ensuring your goals are achieved every step of the way.
Learn more about our project management and our regulatory support
Unleashing the Potential of Nucleic Acid Science
Why Choose BioSpring?
State-of-the-Art Facilities
Our FDA-inspected facilities are designed to optimize capacity utilization and ensure rapid turnaround with high-throughput capabilities, allowing us to support versatile batch requirements for programs targeting both ultrarare and more prevalent diseases. Equipped to manage every aspect of nucleic acid manufacturing and analysis—from small-scale preclinical batches to commercial-scale production—BioSpring brings nearly three decades of experience to ensure your nucleic acid programs are delivered on time and meet the highest quality standards.
Realize the full potential of your genome editing program!
Download our mRNA factsheet now and discover how BioSpring's advanced solutions can take your project to clinical success.
Learn more about our nucleic acid services
The Nucleic Acid Company
About BioSpring
BioSpring is a global leader in nucleic acid manufacturing and analysis, providing comprehensive solutions for therapeutic oligonucleotides (e.g. ASOs and siRNAs) as well as guide RNAs and mRNA. With nearly three decades of experience, we support biotech and pharmaceutical companies at every stage—from early research to commercial production—while maintaining the highest quality standards and ensuring full regulatory compliance.
With a proven track record of supporting hundreds of clinical studies and multiple commercial programs, BioSpring is your trusted partner in advancing nucleic acid-based therapies. Since 2007, we have been cGMP-certified (ICH Q7) for therapeutic oligonucleotide manufacturing and hold ISO 9001 and ISO 13485 certifications for diagnostic oligonucleotides. Our facilities undergo regular inspections by the FDA and other regulatory authorities, guaranteeing compliance with the industry's most stringent standards.






.png?width=729&height=946&name=Factsheet_mRNA_blur%20(1).png)